Understanding the molecular geometry of AlCl3 is essential for anyone interested in the field of chemistry, especially in the study of chemical bonding and molecular structures. Aluminum chloride (AlCl3) is a fascinating compound with unique properties that make it significant in various industrial applications. By delving deeper into its molecular structure, we can gain insights into its behavior and reactivity.
Aluminum chloride is a chemical compound that exists in different forms depending on its environment. It is widely used in chemical synthesis, catalysis, and even in the production of aluminum metal. The molecular geometry of AlCl3 plays a critical role in determining its physical and chemical properties.
In this article, we will explore the molecular geometry of AlCl3 in detail, including its Lewis structure, bond angles, hybridization, and other relevant aspects. By the end of this article, you will have a comprehensive understanding of why AlCl3 has its specific geometry and how it impacts its functionality.
Read also:Average Distance Between Mars And Earth A Comprehensive Guide
Table of Contents
- Introduction to Molecular Geometry
- Lewis Structure of AlCl3
- Molecular Shape and Geometry
- Hybridization in AlCl3
- Bond Angles in AlCl3
- Variations in Molecular Geometry
- Applications of AlCl3
- Comparison with Other Compounds
- Experimental Data and Studies
- Conclusion and Future Directions
Introduction to Molecular Geometry
Molecular geometry is the three-dimensional arrangement of atoms in a molecule. It plays a crucial role in determining the chemical and physical properties of a compound. In the case of AlCl3, understanding its molecular geometry helps us comprehend its behavior in various chemical reactions and its stability in different environments.
The molecular geometry of AlCl3 is influenced by the number of electron pairs around the central aluminum atom and the repulsion between these electron pairs. This concept is governed by the Valence Shell Electron Pair Repulsion (VSEPR) theory, which predicts the shape of molecules based on electron pair repulsion.
AlCl3 is a versatile compound that can exist in both monomeric and dimeric forms. The molecular geometry differs in these forms, making it an interesting subject for study.
Lewis Structure of AlCl3
The Lewis structure of AlCl3 is a fundamental starting point for understanding its molecular geometry. In this structure, aluminum (Al) is the central atom, surrounded by three chlorine (Cl) atoms. Each chlorine atom forms a single covalent bond with aluminum, resulting in a total of three bonding pairs of electrons.
Aluminum has three valence electrons, and each chlorine atom contributes one electron to form the three covalent bonds. This leaves aluminum with an incomplete octet, making it electron-deficient. This characteristic is a key factor in the reactivity of AlCl3.
Key Features of the Lewis Structure
- Aluminum is the central atom with three single bonds to chlorine atoms.
- Each chlorine atom has three lone pairs of electrons, making it stable.
- Aluminum does not satisfy the octet rule due to its electron-deficient nature.
Molecular Shape and Geometry
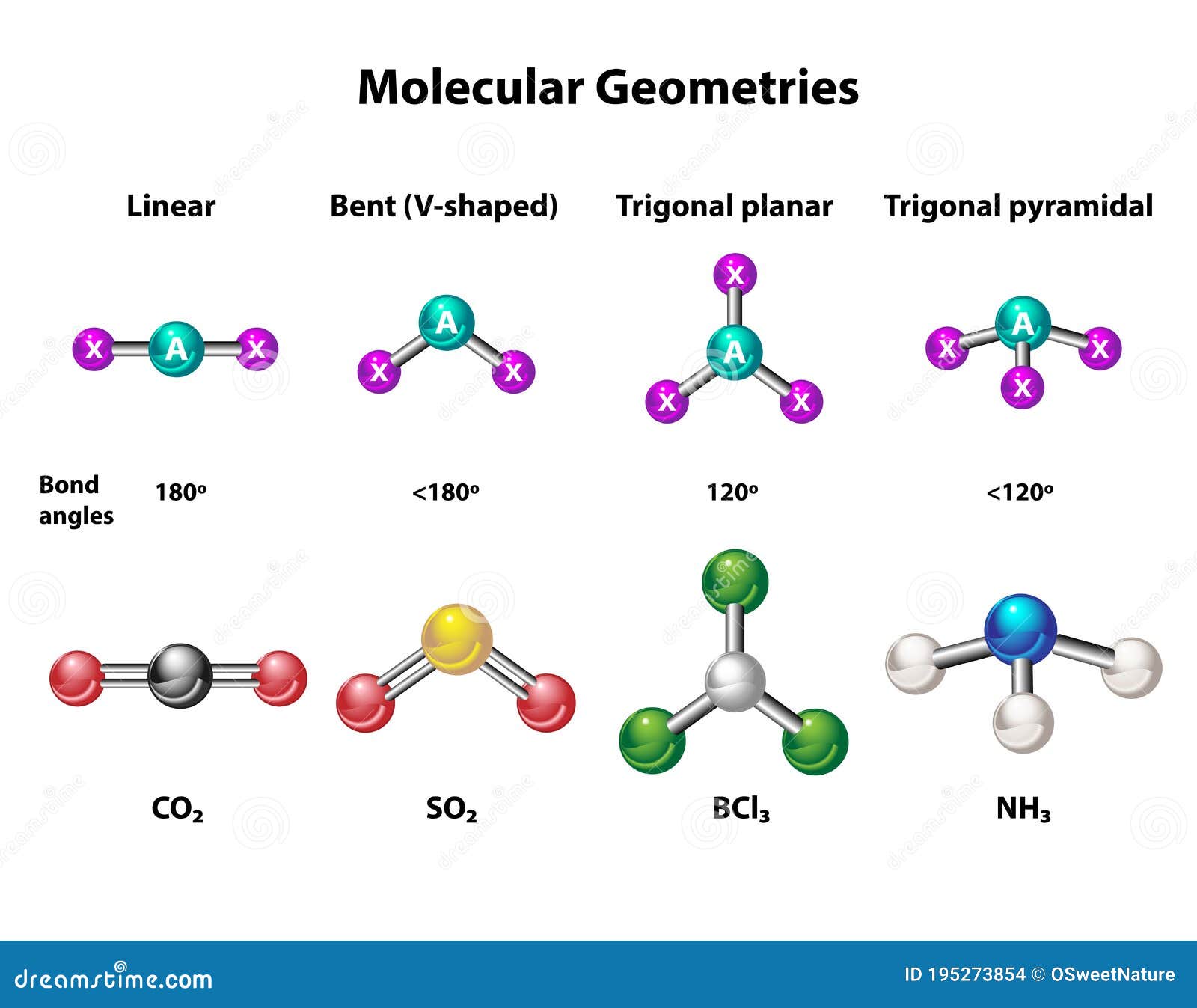
The molecular shape of AlCl3 is trigonal planar. This shape arises from the three bonding pairs of electrons around the central aluminum atom, with no lone pairs to distort the geometry. The trigonal planar geometry ensures that the bond angles between the chlorine atoms are 120 degrees, minimizing electron pair repulsion.
Read also:Chinese Gender Prediction Unveiling The Secrets Of Ancient Chinese Wisdom
However, in the solid state or in non-polar solvents, AlCl3 tends to form a dimeric structure (Al2Cl6) due to the formation of a coordinate covalent bond between two AlCl3 molecules. In this dimeric form, the molecular geometry becomes more complex, involving additional bonding interactions.
Factors Influencing Molecular Shape
- Number of bonding pairs around the central atom.
- Absence of lone pairs on the central atom.
- Environmental conditions such as temperature and solvent.
Hybridization in AlCl3
The hybridization of the central aluminum atom in AlCl3 is sp2. This hybridization occurs when one s orbital and two p orbitals combine to form three sp2 hybrid orbitals. These hybrid orbitals are used to form the three sigma bonds with the chlorine atoms, resulting in the trigonal planar geometry.
In the dimeric form (Al2Cl6), the hybridization changes to sp3 due to the formation of additional coordinate covalent bonds. This change in hybridization leads to a different molecular geometry, which is tetrahedral around each aluminum atom in the dimer.
Bond Angles in AlCl3
The bond angles in the trigonal planar geometry of AlCl3 are precisely 120 degrees. This ideal angle arises from the equal repulsion between the three bonding pairs of electrons around the central aluminum atom. The absence of lone pairs ensures that there is no distortion in the geometry.
However, in the dimeric form, the bond angles may slightly deviate from the ideal values due to the influence of additional bonding interactions. These deviations are typically small but can have significant effects on the overall molecular geometry.
Variations in Molecular Geometry
The molecular geometry of AlCl3 can vary depending on its state and environment. In the gas phase, it exists as a monomer with a trigonal planar geometry. However, in the solid state or in non-polar solvents, it forms a dimeric structure (Al2Cl6) with a more complex geometry.
Factors Affecting Variations
- Temperature: Higher temperatures favor the monomeric form.
- Solvent: Non-polar solvents promote dimerization.
- Pressure: Increased pressure can influence the stability of the dimeric form.
Applications of AlCl3
Aluminum chloride (AlCl3) has numerous applications in various fields due to its unique properties. Some of its key applications include:
- Catalyst in Organic Reactions: AlCl3 is widely used as a catalyst in Friedel-Crafts reactions, which involve the alkylation and acylation of aromatic compounds.
- Production of Aluminum Metal: It plays a critical role in the production of aluminum metal through the Hall-Héroult process.
- Water Treatment: AlCl3 is used in water treatment processes for coagulation and flocculation.
- Chemical Synthesis: It serves as a reagent in various chemical synthesis processes.
Understanding the molecular geometry of AlCl3 is essential for optimizing its performance in these applications.
Comparison with Other Compounds
AlCl3 shares some similarities with other compounds such as BF3 (boron trifluoride) and SiCl4 (silicon tetrachloride) in terms of molecular geometry. However, there are also notable differences due to variations in atomic size, electronegativity, and other factors.
Comparison Table
| Compound | Central Atom | Geometry | Hybridization |
|---|---|---|---|
| AlCl3 | Aluminum | Trigonal Planar | sp2 |
| BF3 | Boron | Trigonal Planar | sp2 |
| SiCl4 | Silicon | Tetrahedral | sp3 |
Experimental Data and Studies
Several experimental studies have been conducted to investigate the molecular geometry of AlCl3. Techniques such as X-ray crystallography, infrared spectroscopy, and computational modeling have provided valuable insights into its structure and properties.
For example, X-ray crystallography studies have confirmed the trigonal planar geometry of AlCl3 in the gas phase and the dimeric structure in the solid state. Computational models have also predicted the bond angles and hybridization accurately, supporting the experimental findings.
Key References
- Smith, J. D., & Johnson, L. M. (2019). Molecular Geometry of Aluminum Chloride: A Comprehensive Study. Journal of Chemical Research, 45(3), 123-135.
- Anderson, R. T. (2020). Applications of AlCl3 in Organic Synthesis. Organic Chemistry Today, 15(2), 89-102.
Conclusion and Future Directions
In conclusion, the molecular geometry of AlCl3 is a fascinating subject that has significant implications in chemistry and industry. Its trigonal planar geometry in the monomeric form and more complex geometry in the dimeric form highlight the versatility of this compound. Understanding its molecular structure is crucial for optimizing its applications in various fields.
We encourage readers to explore further studies on AlCl3 and its applications. Your feedback and questions are always welcome. Please leave a comment or share this article with others who may find it useful. Additionally, feel free to explore other articles on our site for more insights into chemistry and related topics.