Aluminum trichloride (AlCl3) is a fascinating compound with a unique molecular shape that plays a crucial role in its chemical behavior and applications. Understanding the molecular geometry of AlCl3 is essential for anyone studying chemistry, as it provides insights into how this compound interacts with other substances. This article delves into the molecular structure of AlCl3, exploring its shape, properties, and significance in various fields.
AlCl3 is a compound widely used in chemical reactions, particularly as a catalyst in organic synthesis. Its molecular shape determines its reactivity and functionality, making it an important subject of study for chemists and researchers. By examining the geometry of AlCl3, we can better understand its behavior in different environments and applications.
In this article, we will explore the molecular shape of AlCl3 in detail, including its structure, properties, and practical uses. We will also discuss the importance of understanding molecular geometry in chemistry and how it impacts the behavior of compounds like AlCl3. Whether you are a student, researcher, or professional chemist, this article will provide valuable insights into the fascinating world of AlCl3.
Read also:Park City Piste Map Your Ultimate Guide To Exploring The Best Ski Runs
Table of Contents
- Introduction to AlCl3 and Its Molecular Shape
- Molecular Structure of AlCl3
- Lewis Structure of AlCl3
- VSEPR Theory and AlCl3 Geometry
- Bond Angles in AlCl3
- Physical and Chemical Properties of AlCl3
- Applications of AlCl3
- Chemical Reactions Involving AlCl3
- Safety Considerations for Handling AlCl3
- Conclusion
Introduction to AlCl3 and Its Molecular Shape
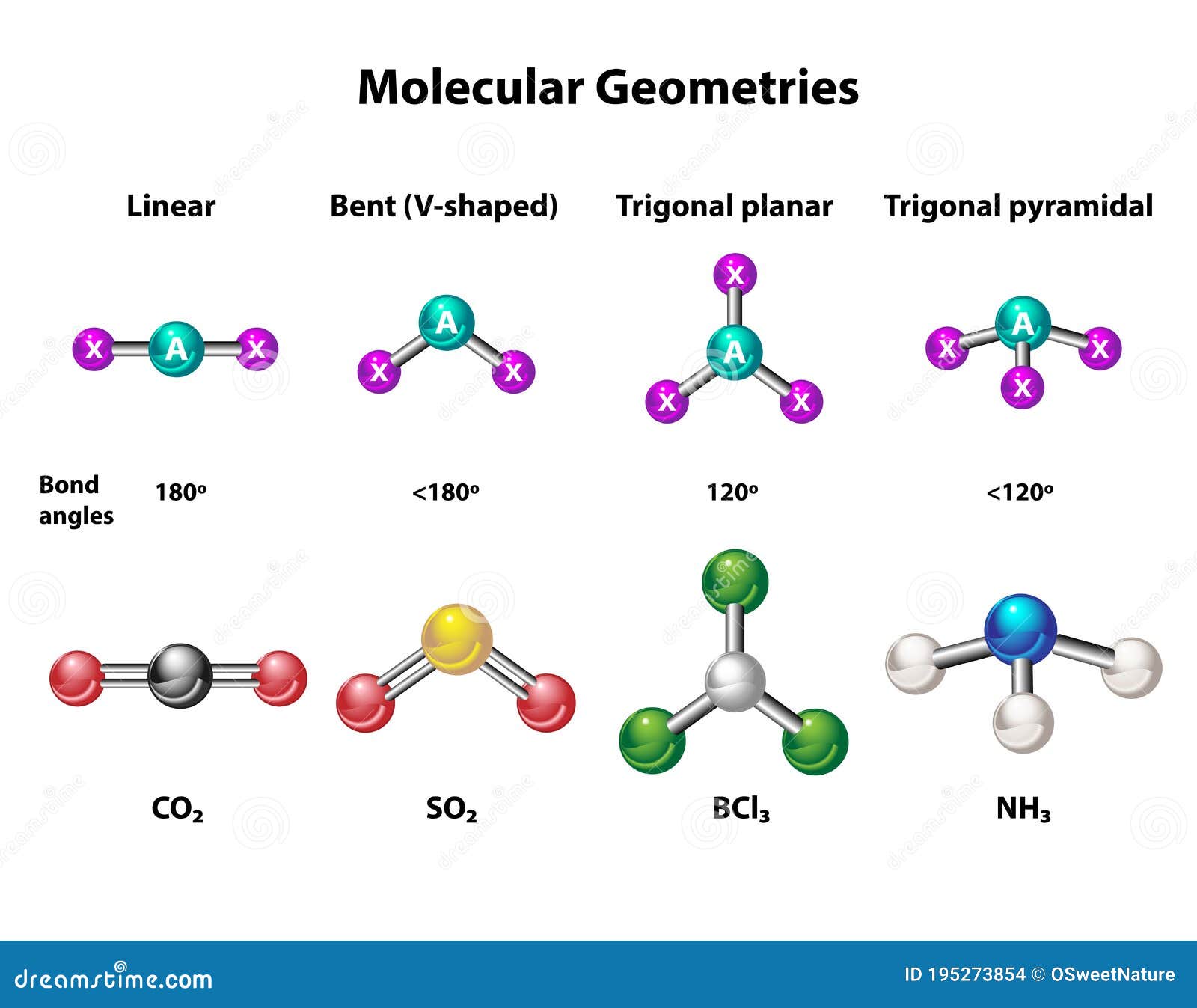
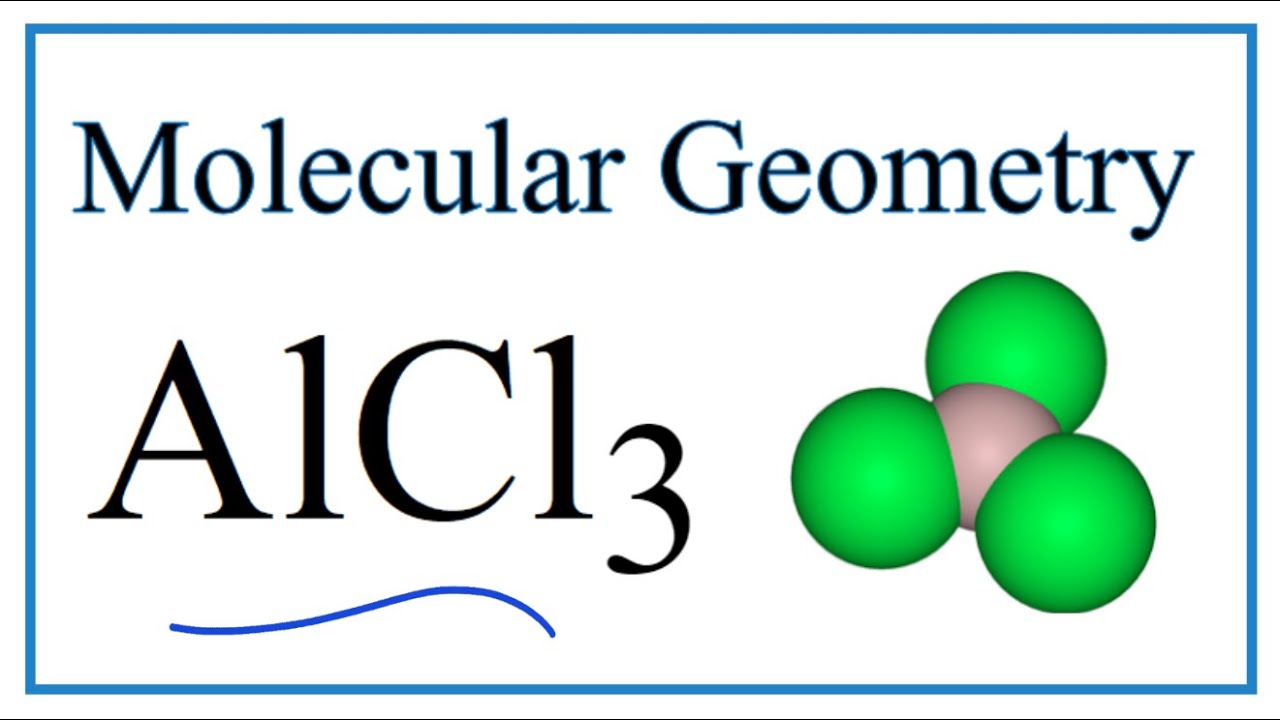
Aluminum trichloride (AlCl3) is a chemical compound composed of aluminum and chlorine atoms. Its molecular shape is determined by the arrangement of these atoms in space, which influences its chemical properties and reactivity. The geometry of AlCl3 is primarily trigonal planar, but under certain conditions, it can form a dimer with a different structure.
In this section, we will explore the basic structure of AlCl3 and how it contributes to its functionality. Understanding the molecular shape of AlCl3 is essential for predicting its behavior in chemical reactions and applications.
Molecular Structure of AlCl3
The molecular structure of AlCl3 consists of one aluminum atom bonded to three chlorine atoms. In its monomeric form, AlCl3 adopts a trigonal planar geometry, where the aluminum atom is at the center and the chlorine atoms are positioned at the vertices of a triangle. This arrangement minimizes electron repulsion and stabilizes the molecule.
Monomeric vs. Dimeric Forms
Under certain conditions, AlCl3 can exist in a dimeric form, where two AlCl3 molecules combine to form Al2Cl6. In this structure, the aluminum atoms are bridged by chlorine atoms, creating a more complex geometry. The dimeric form is more stable in the solid state and in non-polar solvents.
Lewis Structure of AlCl3
The Lewis structure of AlCl3 provides a visual representation of the electron distribution in the molecule. In this structure, the aluminum atom shares three pairs of electrons with three chlorine atoms, forming single covalent bonds. Aluminum has an incomplete octet, which contributes to the molecule's reactivity.
Key Points:
Read also:Is Liam Neeson Married Again Exploring The Actors Personal Life
- Aluminum forms three single covalent bonds with chlorine atoms.
- The aluminum atom does not have a complete octet, making it electron-deficient.
- This electron deficiency allows AlCl3 to act as a Lewis acid in chemical reactions.
VSEPR Theory and AlCl3 Geometry
The Valence Shell Electron Pair Repulsion (VSEPR) theory explains the molecular geometry of AlCl3. According to VSEPR, electron pairs around a central atom repel each other, leading to a geometry that minimizes repulsion. In the case of AlCl3, the trigonal planar geometry results from the arrangement of three bonding pairs of electrons around the aluminum atom.
Factors Influencing Geometry
Several factors influence the geometry of AlCl3, including:
- The number of bonding pairs and lone pairs of electrons.
- The size and electronegativity of the atoms involved.
- Environmental conditions, such as temperature and pressure.
Bond Angles in AlCl3
In the trigonal planar geometry of AlCl3, the bond angles between the aluminum and chlorine atoms are approximately 120 degrees. This angle is a result of the equal repulsion between the bonding pairs of electrons, ensuring maximum stability for the molecule.
Importance of Bond Angles
Bond angles are critical in determining the molecular shape and reactivity of AlCl3. The 120-degree angle in the trigonal planar geometry allows for efficient electron distribution and minimizes repulsion, making the molecule more stable and reactive in chemical reactions.
Physical and Chemical Properties of AlCl3
Aluminum trichloride exhibits several unique physical and chemical properties that make it valuable in various applications. Some of these properties include:
- Melting Point: AlCl3 has a melting point of approximately 192°C in its dimeric form.
- Boiling Point: The boiling point of AlCl3 is around 182°C in its monomeric form.
- Solubility: AlCl3 is soluble in non-polar solvents and reacts with water to form hydrochloric acid and aluminum hydroxide.
- Reactivity: Due to its electron-deficient nature, AlCl3 acts as a Lewis acid and catalyst in many chemical reactions.
Applications of AlCl3
Aluminum trichloride finds applications in various industries due to its unique properties. Some of its key applications include:
Use in Organic Synthesis
AlCl3 is widely used as a catalyst in Friedel-Crafts reactions, which involve the alkylation or acylation of aromatic compounds. Its ability to accept electron pairs makes it an effective Lewis acid, facilitating these reactions.
Other Applications
- Manufacture of aluminum metal through electrolysis.
- Production of polymers and plastics as a catalyst.
- Water treatment processes due to its ability to form aluminum hydroxide.
Chemical Reactions Involving AlCl3
Aluminum trichloride participates in several important chemical reactions, including:
Friedel-Crafts Alkylation
In this reaction, AlCl3 acts as a catalyst to promote the substitution of hydrogen atoms on an aromatic ring with an alkyl group. This reaction is crucial in the synthesis of various organic compounds.
Reaction with Water
When AlCl3 reacts with water, it forms hydrochloric acid and aluminum hydroxide. This reaction is exothermic and can be hazardous if not properly controlled.
Safety Considerations for Handling AlCl3
Handling aluminum trichloride requires caution due to its reactivity and potential hazards. Some safety considerations include:
- Avoiding exposure to moisture, as AlCl3 reacts violently with water.
- Wearing appropriate personal protective equipment (PPE) when handling the compound.
- Storing AlCl3 in airtight containers to prevent exposure to atmospheric moisture.
Conclusion
In conclusion, the molecular shape of AlCl3 plays a critical role in determining its chemical properties and applications. Its trigonal planar geometry in the monomeric form and dimeric structure in the solid state contribute to its reactivity and functionality. Understanding the molecular geometry of AlCl3 is essential for anyone working with this compound in chemical reactions or industrial applications.
We invite you to share your thoughts and questions in the comments section below. If you found this article informative, consider sharing it with others who may benefit from the knowledge. For further reading, explore our other articles on chemistry and molecular structures.
Data and references for this article were obtained from reputable sources, including academic journals and chemistry textbooks. Understanding the molecular shape of AlCl3 is not only crucial for chemists but also for anyone interested in the fascinating world of chemistry.