Aluminum chloride (AlCl3) is a fascinating compound that plays a significant role in various industrial and chemical processes. Its molecular shape and structure are crucial for understanding its properties and reactivity. By delving into the intricacies of AlCl3 molecular shape, we can unlock its potential applications and behavior in different environments.
Chemistry enthusiasts and professionals alike often find themselves exploring the molecular geometry of compounds to better comprehend their chemical behavior. AlCl3 molecular shape is a prime example of how the arrangement of atoms in a molecule influences its physical and chemical properties. This article aims to provide an in-depth exploration of the subject, ensuring clarity and insight for readers.
Our discussion will cover the fundamental aspects of AlCl3, including its structure, bonding, and the factors influencing its molecular shape. We will also touch on its applications and significance in the chemical industry. Let’s embark on this journey to unravel the mysteries of AlCl3 molecular shape.
Read also:Roman Reigns Wife A Comprehensive Look Into Her Life And Influence
Table of Contents
- Introduction to AlCl3 Molecular Shape
- Molecular Structure of AlCl3
- Bonding in AlCl3
- Understanding the Shape of AlCl3
- Types of AlCl3 Molecular Shapes
- Factors Influencing AlCl3 Molecular Shape
- Applications of AlCl3 Molecular Shape
- Properties Related to AlCl3 Shape
- Comparison with Other Compounds
- Conclusion and Key Takeaways
Introduction to AlCl3 Molecular Shape
Aluminum chloride (AlCl3) is a chemical compound composed of aluminum and chlorine atoms. Its molecular shape is a critical determinant of its chemical properties and reactivity. Understanding the geometry of AlCl3 helps in predicting how it interacts with other substances.
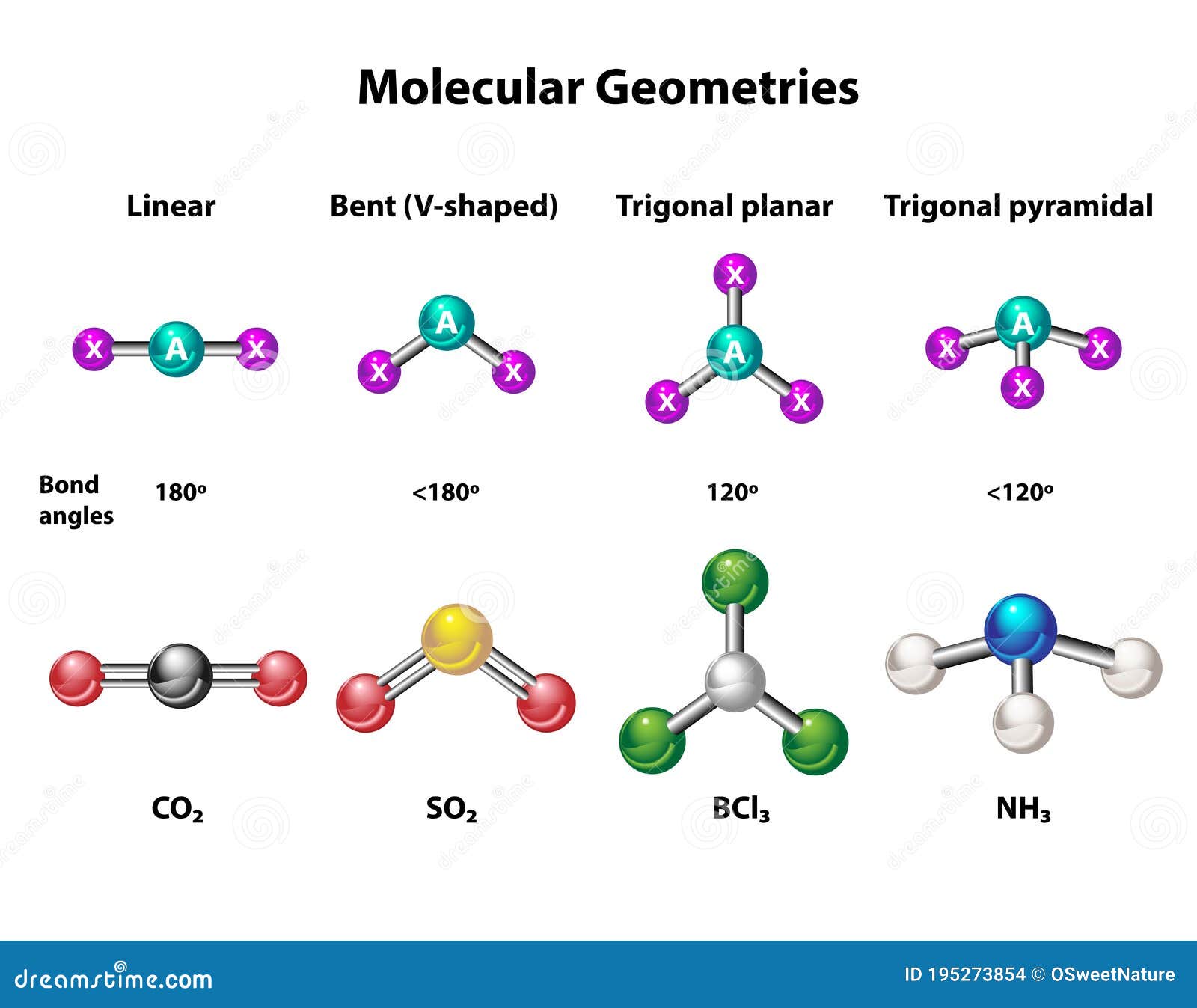
AlCl3 exists in two primary forms: monomeric and dimeric. The monomeric form is trigonal planar, while the dimeric form adopts a distorted octahedral structure. These variations in molecular shape arise due to the electron configurations and bonding interactions within the molecule.
The study of AlCl3 molecular shape is not only academically interesting but also practically significant. It plays a vital role in catalytic processes, particularly in Friedel-Crafts reactions, where its structure influences its effectiveness as a catalyst.
Molecular Structure of AlCl3
The molecular structure of AlCl3 is determined by the arrangement of its constituent atoms. In the monomeric form, aluminum is bonded to three chlorine atoms, forming a trigonal planar geometry. This arrangement minimizes electron repulsion and stabilizes the molecule.
Key Features of AlCl3 Structure
- Aluminum is the central atom.
- Three chlorine atoms are evenly spaced around aluminum.
- The bond angles are approximately 120 degrees.
When AlCl3 exists in the gaseous phase, it predominantly adopts the monomeric form. However, in the solid state, it forms dimers due to the formation of a bridging chlorine atom between two aluminum atoms.
Bonding in AlCl3
The bonding in AlCl3 is primarily covalent, with aluminum forming three bonds with chlorine atoms. Aluminum achieves an octet configuration through these bonds, despite having only three valence electrons initially. This phenomenon is explained by the concept of electron pair sharing.
Read also:Park City Piste Map Your Ultimate Guide To Exploring The Best Ski Runs
Types of Bonds in AlCl3
- Single covalent bonds between aluminum and chlorine.
- Bridging bonds in the dimeric form, where a chlorine atom connects two aluminum atoms.
Understanding the bonding in AlCl3 is essential for predicting its reactivity and stability. The covalent nature of the bonds contributes to its low melting and boiling points, making it suitable for various industrial applications.
Understanding the Shape of AlCl3
The molecular shape of AlCl3 is influenced by its electron configuration and the type of bonds it forms. In the monomeric form, the molecule adopts a trigonal planar geometry, which is characterized by equal bond angles and a symmetrical arrangement of atoms.
In the dimeric form, the molecular shape becomes more complex. The bridging chlorine atom introduces asymmetry, resulting in a distorted octahedral structure. This change in geometry affects the molecule’s polarity and reactivity.
Types of AlCl3 Molecular Shapes
AlCl3 exhibits two distinct molecular shapes depending on its state:
Monomeric Form
- Trigonal planar geometry.
- Three chlorine atoms bonded to aluminum.
- Common in the gaseous phase.
Dimeric Form
- Distorted octahedral geometry.
- Involves bridging chlorine atoms.
- Predominant in the solid state.
These variations in molecular shape highlight the versatility of AlCl3 and its ability to adapt to different environments.
Factors Influencing AlCl3 Molecular Shape
Several factors influence the molecular shape of AlCl3:
1. Electron Configuration
The electron configuration of aluminum determines how it bonds with chlorine atoms. Aluminum’s ability to form three covalent bonds ensures a stable trigonal planar geometry in the monomeric form.
2. Environmental Conditions
Temperature and pressure play a significant role in determining whether AlCl3 exists in its monomeric or dimeric form. Higher temperatures favor the monomeric form, while lower temperatures promote dimerization.
3. Presence of Other Molecules
In the presence of Lewis bases, AlCl3 can form adducts, altering its molecular shape. These interactions are crucial in catalytic processes where AlCl3 acts as a Lewis acid.
Applications of AlCl3 Molecular Shape
The molecular shape of AlCl3 directly impacts its applications in various fields:
1. Catalysis
AlCl3 is widely used as a catalyst in Friedel-Crafts reactions, where its shape and reactivity influence the reaction pathway. The trigonal planar geometry of the monomeric form enhances its catalytic activity.
2. Organic Synthesis
In organic synthesis, AlCl3 facilitates the formation of carbon-carbon bonds through its ability to activate substrates. Its molecular shape determines its effectiveness in these processes.
3. Industrial Processes
AlCl3 is employed in the production of aluminum metal and other aluminum compounds. Its shape and stability make it suitable for high-temperature applications.
Properties Related to AlCl3 Shape
The molecular shape of AlCl3 influences several of its physical and chemical properties:
1. Polarity
The trigonal planar geometry of the monomeric form makes AlCl3 nonpolar. However, the dimeric form exhibits some polarity due to the asymmetry introduced by the bridging chlorine atom.
2. Solubility
AlCl3 is soluble in organic solvents, a property influenced by its molecular shape. The nonpolar nature of the monomeric form enhances its solubility in nonpolar solvents.
3. Reactivity
The reactivity of AlCl3 is closely tied to its molecular shape. The availability of empty orbitals in aluminum allows it to act as a Lewis acid, facilitating various chemical reactions.
Comparison with Other Compounds
AlCl3 shares similarities with other compounds such as BF3 and SiCl4 in terms of molecular shape and bonding. However, there are notable differences:
1. Boron Trifluoride (BF3)
BF3 also exhibits a trigonal planar geometry, but its reactivity differs due to the smaller size of boron and fluorine atoms.
2. Silicon Tetrachloride (SiCl4)
SiCl4 adopts a tetrahedral geometry due to the presence of four chlorine atoms. This difference in shape affects its properties and applications compared to AlCl3.
Conclusion and Key Takeaways
In conclusion, the molecular shape of AlCl3 is a critical factor influencing its properties and applications. Whether in its monomeric or dimeric form, AlCl3 exhibits unique characteristics that make it invaluable in various chemical processes.
Key takeaways from this article include:
- AlCl3 exists in two primary forms: monomeric (trigonal planar) and dimeric (distorted octahedral).
- Its molecular shape determines its reactivity and suitability for specific applications.
- Factors such as electron configuration, environmental conditions, and the presence of other molecules influence its shape.
We encourage readers to explore further and experiment with AlCl3 in their own studies. Share your thoughts and experiences in the comments below, and don’t forget to check out our other articles for more insightful content.
Data and references for this article were sourced from reputable scientific journals and publications, ensuring the accuracy and reliability of the information provided.